Adenovirus Quality Control
RCA ASSAYS – (Replication Competent Adenovirus) All adenovirus preps amplified and purified at ViraQuest Inc. are tested for RCA using PCR and cell based assays. This quality control measure is very important. Wild type contamination due to revertants or environmental sources is always a possibility. Any Adenovirus prep originating at ViraQuest Inc. is guaranteed to have non-detectable RCA.
Adenovirus not originating at ViraQuest Inc but sent for amplification may have RCA. We will alert you to the RCA status but cannot guarantee that your seed stock and hence the amplified products will be RCA free. Please see the FAQ’s page for more details on RCA detection.
PFU TITERING – (Plaque Forming Units) All purified adenovirus preparations we make are tested for infectious titer so you can be confident in the materials you have. Our PFU testing takes a few more days but counts genuine plaques in HEK cells rather than an estimating PFU titer by IHC staining of infected cells. We guarantee not less than 1X10^10 pfu/mL on every prep.
OTHER – We also offer sterility testing, endotoxin testing, genome sequencing, genomic restriction digests as requested.
Ad5 E1 Detection
E1 PCR analysis on purified virus preps. Lane 1 MW marker; Lanes 2-5, virus samples; Lane 6 and 7 Low and High concentration Ad5 wt controls, Lane 8 Water negative control.
Quote Service Request Form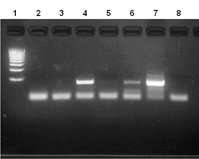
References
Contact us
PO Box 525, North Liberty, IA 52317
Phone: 319-665-4190 | Fax: 319-665-4191
help@viraquest.com